


[Allergen-specific IgE Antibody Detection Kit for Set 1 (Inhalation and Food) Allergens (Fluorescence Immunoassay)] Officially Approved!
On October 11, 2025, the Allergen-specific IgE Antibody Detection Kit for Set 1 (Inhalation and Food) Allergens (Fluorescence Immunoassay) developed by Suzhou Coninno Biotechnology Co., Ltd. obtained the Class Ⅱ Medical Device Registration Certificate from the Jiangsu Products Administration!11
2025-10
Fluorescent Immunochromatographic Analyzer Officially Approved!
On September 2, 2025, the Fluorescent Immunochromatographic Analyzer developed by Suzhou Coninno Biotechnology Co., Ltd. obtained the Class Ⅱ Medical Device Registration Certificate from the Jiangsu Products Administration!02
2025-09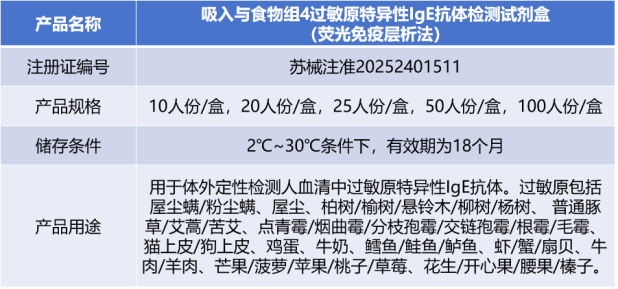
[Allergen-specific IgE Antibody Detection Kit for Set 4 (Inhalation and Food) Allergens (Fluorescence Immunoassay)] Officially Approved!
On August 1, 2025, the Allergen-specific IgE Antibody Detection Kit for Set 4 (Inhalation and Food) Allergens (Fluorescence Immunoassay) developed by Suzhou Coninno Biotechnology Co., Ltd. obtained the Class Ⅱ Medical Device Registration Certificate from the Jiangsu Products Administration!01
2025-08
[Allergen-specific IgE Antibody Detection Kit for Set 3 (Inhalation and Food) Allergens (Fluorescence Immunoassay)] Officially Approved!
On July 31, 2025, the Allergen-specific IgE Antibody Detection Kit for Set 3 (Inhalation and Food) Allergens (Fluorescence Immunoassay) developed by Suzhou Coninno Biotechnology Co., Ltd. obtained the Class Ⅱ Medical Device Registration Certificate from the Jiangsu Products Administration!31
2025-07
[Sample Diluent] Officially Approved!
On July 4, 2025, the Sample Diluent developed by Suzhou Coninno Biotechnology Co., Ltd. obtained the Medical Device Filing Certificate from the Jiangsu Provincial Medical Products Administration!04
2025-07
About Us
Product Center
News Center
Application Scenarios
Contact Us
COPYRIGHT 2025 Coninno Biotechnology All rights reserved 苏ICP备2025158326号-1 (X)网药械信息备字(XXXX)第 XXXX 号
Online Map